Research
We study insect-parasitic nematodes and their mutualist bacterial partners to address a variety of conceptual areas.
Evolution of Parasite Virulence and Life History
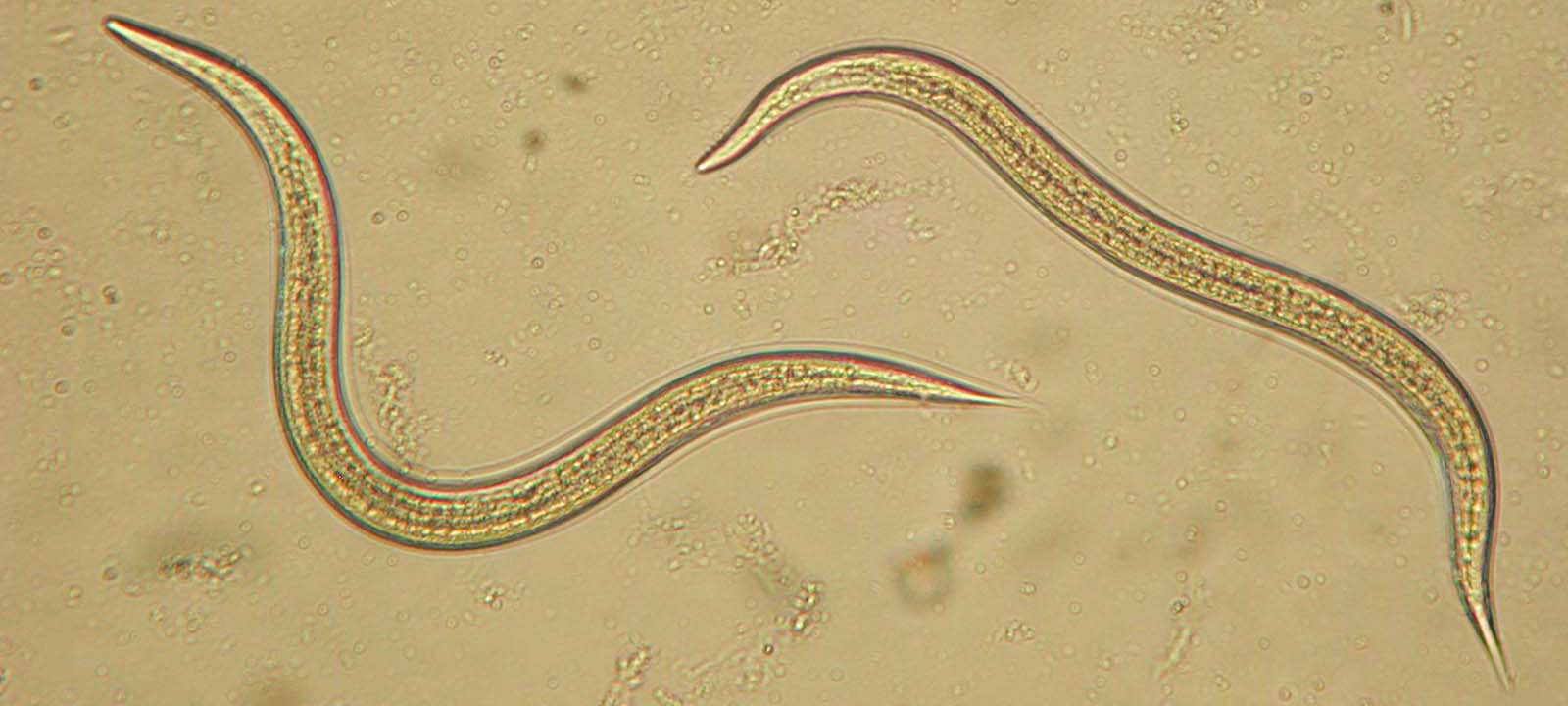
Parasite virulence has been modeled as a life-history problem, where parasites trade-off increased reproduction with lowered transmission success. When multiple parasites infect a host, the behavior of one parasite can influence the success of other co-infecting parasites. Thus, co-infections are predicted to have a profound impact on the evolution virulence.  We were able to provide one of the first experimental tests of this theory, using the insect-parasitic nematode, Steinernema carpocapsae. We took an experimental evolution approach, manipulating migration to influence the relatedness of parasites co-infecting an insect host.
We were able to provide one of the first experimental tests of this theory, using the insect-parasitic nematode, Steinernema carpocapsae. We took an experimental evolution approach, manipulating migration to influence the relatedness of parasites co-infecting an insect host.
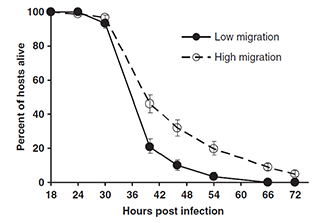
We found that restricted migration led to faster host exploitation and more rapid host death. We hypothesized that interference competition (such as bacteriocin-mediated antagonism) among unrelated parasites can reduce their ability to use host resources, and thus lessen the detrimental effect that they had on their hosts. That is, unrelated parasites fight each other (at the expense of their ability to exploit their host), while related parasites “cooperate” by not fighting.
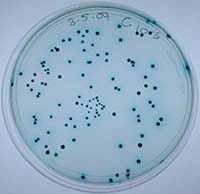
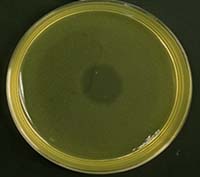
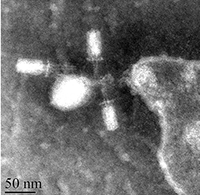
We tested this hypothesis by isolating the Xenorhabdus bacteria from insects that had been killed by parasites from our low- and high-migration lines. We found inhibitions within hosts infected with parasites from the high-migration treatment, but none within hosts in the low-migration treatment. Our study was the first to document intraspecific antagonism in X. nematophila. Furthermore, it was the first empirical demonstration that parasite migration can affect within-host interference competition and virulence.

In addition to our work focusing on within-host competition, we have also examined the potential for among-host selection in the nematode S. carpocapsae. Selection among hosts is thought to be an important force shaping the evolution of parasite life histories; nevertheless, few studies have empirically examined among-host selection. We selected among insect hosts by propagating nematodes based on the total number of nematodes emerging from an insect. We found a direct response to this group-level selection as a forty percent difference in population size evolved between the low- and high-selected treatments. Moreover, selection on population size in our experiment affected the timing of nematode emergence and the size of emerging nematodes. The size of emerging nematodes is likely to influence their fitness, as they are non-feeding until they find a new host. Furthermore, we have found that large nematodes have a higher infection success than small nematodes and that aging in the transmission stage can alter species interactions. These results suggest that it is critical to examine both within and among-host selection in order to understand infection dynamics and the evolution of parasite life histories.